





The Certification Checklist: Understanding FDA, CE, and ISO Standards in Dental Aligners
Clear aligners may look like simple pieces of plastic, but each tray is a regulated medical device. Whether a practice orders trays from a global manufacturer or a local lab, three quality marks should always appear on the packaging and paperwork: FDA clearance (USA), CE marking (Europe) and ISO 13485 certification (global).
Below is a plain-language guide to what each approval really means, plus a quick checklist for keeping your practice and your patients safe.


FDA 510(k) Clearance: “Proven to Be As Safe and Effective”
Scope – The U.S. Food & Drug Administration reviews technical data to show the aligner system is substantially equivalent to a legally marketed predicate.
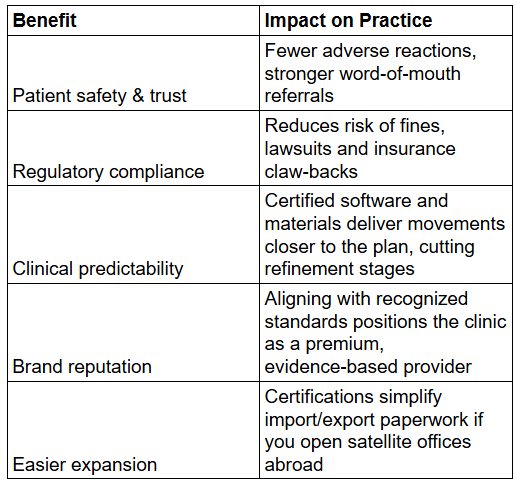
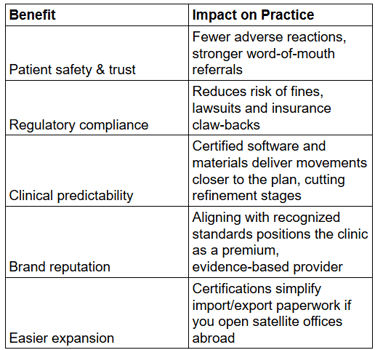
Why it matters – FDA clearance confirms biocompatibility (no hidden toxins), manufacturing consistency, and digital treatment-planning software safety. Using a non-cleared product in the U.S. exposes dentists to enforcement action and insurance rejection.
What to look for – Ask the supplier for their 510(k) number and verify it in the FDA’s public database; ensure the software and the plastic material are both covered.
CE Mark Under the EU MDR: “Free to Move Across Europe”
Scope – The CE mark shows the device meets the EU Medical Device Regulation (MDR) for safety, performance and post-market surveillance.
Why it matters – CE marking is accepted in many Middle-East and African markets as proof of conformity. It also enforces traceability, meaning every batch of trays can be tracked back to its raw material lot—vital when field-corrective actions are needed.
What to look for – The CE logo must be followed by a four-digit Notified-Body number (e.g., CE 2797). Ask for a current Declaration of Conformity that lists the aligner and the tooth-movement software.
ISO 13485 Quality-Management Certification: “Process You Can Trust”
Scope – ISO 13485 is an international standard that audits how the manufacturer designs, produces, tests and services medical devices.
Why it matters – A plant with ISO 13485 has formalized risk management, document control and supplier qualification. That translates into fewer fit issues, easier mid-course corrections and predictable lead times for your practice.
What to look for – Request the latest ISO 13485 certificate and check its expiry date and issuing body; reputable firms undergo annual surveillance audits.




Quick-Check Protocol for Your Team
Verify certificates before signing a supply contract check FDA number, CE Declaration, ISO 13485.
Log and trace each batch/lot number in the patient’s chart.
Inspect incoming trays for CE and lot labels; quarantine any missing marks.
Request the SDS (Safety Data Sheet) for the plastic resin to reassure sensitive patients.
Schedule supplier reviews annually; certifications can lapse or be suspended.
Educate staff & patients—display the certificates in-office and on your website.
If you need more information then do not hesitate to contact us. Send us an email at info@alignersconsultancy.com
